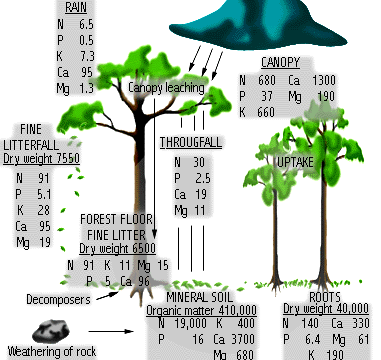
would really like to see your data on the high amount of K in rain water.
Rick, I was trying to find the relevent article but trudging through the endless maze of Nature's archive is not my idea of fun. From the small exerpt that I could find the idea was basically that there exists a very thin layer on the ocean surface which contain significant levels of K and other elements as a result of micro organism activity and that this layer can be picked up by wind off the white caps and transfered to the atmosphere resuting in readings of K levels in the resulting rain as high as 10 times that found in bulk sea water. But we are really starting to drift off on a tangent once again.
The point being that whether from dust or ocean spray rain can carry and deposit nutrients. So why is this K not accumulating in the leaves of rainforest trees when we know that they will do so given the opportunity?
One explanation could be that there is a higher level of Ca. in the system but I was under the impression that plants always take up K in preference to Ca.
If you feed equal parts K and Ca, (in an inert media) a leaf analysis would show K above Ca.
If you feed Ca. at higher levels you would begin to see K deficiency.
Your levels of very low or no K would probably result in K deficiency to anyone growing paphs in S/H. Therefore we need to have some idea how much inherant K is in the growing mix.
As you have pointed out, bark, fern fiber, chc, and leaves have enough K present to not require any addition for probably 12 months. After that, levels will start drop to a time when K will need to be added. So how much do you add? Leaving Ca and Mg. aside for a moment, my data says at the VERY LEAST K/N ratio should be 50%K to N for flowering plants. Your data probably would say something more like 10%
K to N? or 100ppm N and 10ppm K. would that be about right?
I enjoy reading your postings and respect your experience and observations in growing paphs. And I'm trialing your low K regimen based on your findings.
It's all very interesting stuff but with so much conflicting information out there, I feel the need to question everything. I hope you don't mind that.
I guess that's the purpose of this forum

Mike