Rick
Well-Known Member
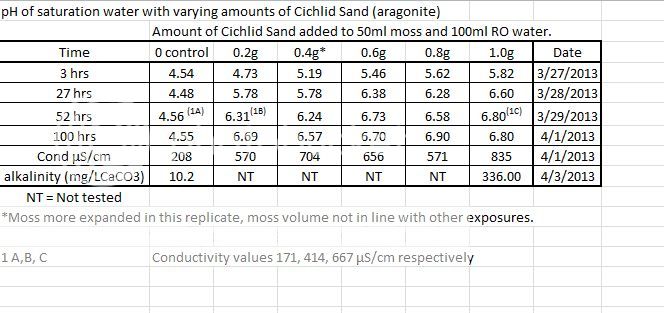
Ok here's some results from a trial I did with adding different amounts of CaribSea Cichlid Sand (aragonite sand, aragonite is almost pure calcium carbonate) to a fixed amount of NZ sphagnum moss in 100ml of RO water.
The amount of moss was the hardest to control. I compressed as much dry moss as I could pack into a 50mL measuring scoop, but after going into water and expanding, I could see that the 0.4gr exposure had more moss than the other exposures.
I was surprised that even the lowest dose of sand (only 0.2 grams) was more than enough to buffer the moss pH into optimal fertilizer uptake range, and actually put the alkalinity and conductivity (TDS) beyond what I would want to keep my plants in long term.



